Mechanics of DNA
Faculty Participants
Catalin Picu
Professor
Current and Former Students
Ehsan Ban
Graduate Research Assistant
Mechanics of Self Assembled DNA Crystals
3D DNA crystals have important potential applications in nanotechnology, for example in mediating the orientation of nanostructures in arrays and trapping biomolecules for crystallographic structure identification.Some crystals of this type aremade up from triangular structures attached at their corners as shown in Fig. 1. The junctions are similar to Holliday junctions and the crystal has rhombohedral symmetry. (More at Ned Seeman’s Homepage)
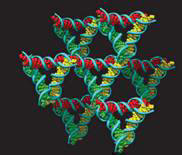
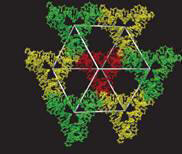
Mechanics of Double Stranded DNA
Classical Molecular Dynamics is used to study the deformation and failure of such structures at ambient temperature.
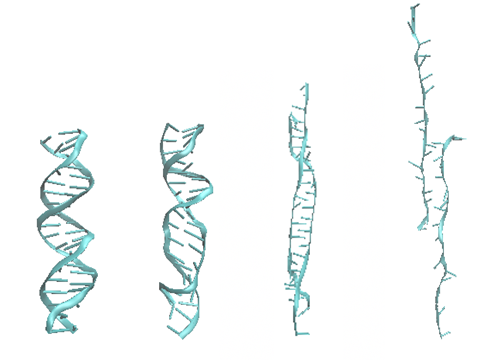
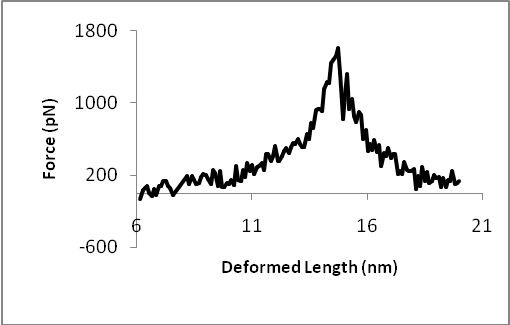
Let us look first at how an isolated DNA double strand deforms. Figure 2 shows the force-displacement relation and four snapshots of the simulation. The peak of the curve corresponds to the third configuration. The last configuration is selected from the right side of the curve, corresponding to a smaller force.
Mechanics of Sticky Ends
Since the crystal is assembled from strands which pair at their ends (“sticky ends”), it is interesting to simulate the deformation and failure of a double strand containing a sticky end. Such a model is obtained by introducing nicks in the opposite strands of a perfect DNA double helix. The resulting molecule is stretched in the same fashion as the double stranded DNA.The deformed structure and the force-displacement curve are shown in Figure 3.
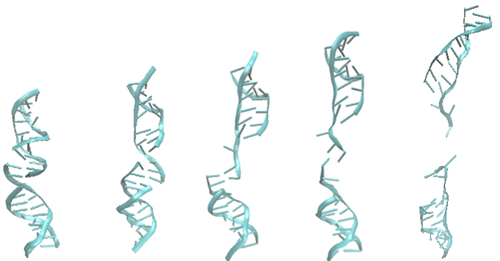
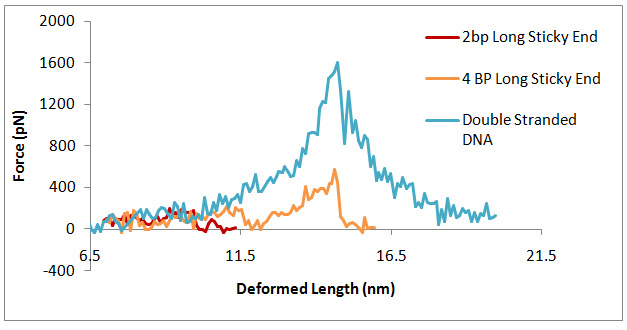
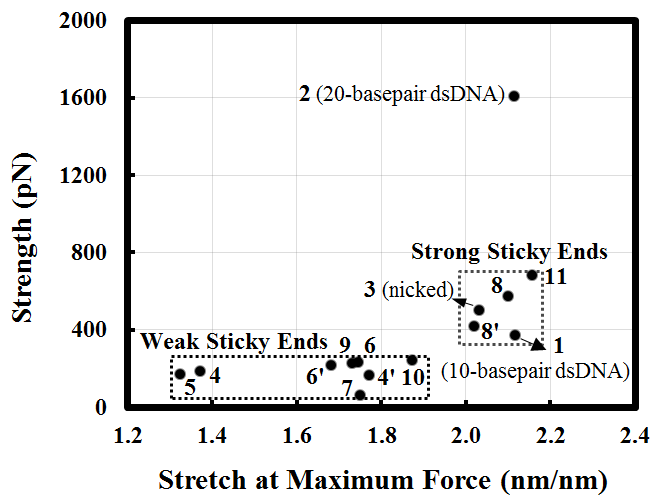
Figure 4 shows the strength of sticky ends computed for the listed structures. Figure 4(a) shows the connected DNA strands, with the sticky end sequence being marked with vertical bars. Figure 4(b) shows the dissociation strengths evaluated in uniaxial tension for the structures in Figure 4(a). These can be classified in “strong” and “weak” as indicated.
 a)
a)
 b)
b)
References
- [1]J. Zheng et al., “From molecular to macroscopic via the rational design of a self-assembled 3D DNA crystal,” Nature, vol. 461, no. 7260, pp. 74–77, Sep. 2009, doi: 10.1038/nature08274.
We live in a macroscopic three-dimensional (3D) world, but our best description of the structure of matter is at the atomic and molecular scale. Understanding the relationship between the two scales requires a bridge from the molecular world to the macroscopic world. Connecting these two domains with atomic precision is a central goal of the natural sciences, but it requires high spatial control of the 3D structure of matter. The simplest practical route to producing precisely designed 3D macroscopic objects is to form a crystalline arrangement by self-assembly, because such a periodic array has only conceptually simple requirements: a motif that has a robust 3D structure, dominant affinity interactions between parts of the motif when it self-associates, and predictable structures for these affinity interactions. Fulfilling these three criteria to produce a 3D periodic system is not easy, but should readily be achieved with well-structured branched DNA motifs tailed by sticky ends. Complementary sticky ends associate with each other preferentially and assume the well-known B-DNA structure when they do so; the helically repeating nature of DNA facilitates the construction of a periodic array. It is essential that the directions of propagation associated with the sticky ends do not share the same plane, but extend to form a 3D arrangement of matter. Here we report the crystal structure at 4 Å resolution of a designed, self-assembled, 3D crystal based on the DNA tensegrity triangle. The data demonstrate clearly that it is possible to design and self-assemble a well-ordered macromolecular 3D crystalline lattice with precise control.
Publications
- [1]E. Ban and C. R. Picu, “Strength of DNA Sticky End Links,” Biomacromolecules, vol. 15, no. 1, pp. 143–149, Jan. 2014, doi: 10.1021/bm401425k.
Sticky ends are unpaired nucleotides at the ends of DNA molecules that can associate to link DNA segments. Self-assembly of DNA molecules via sticky ends is currently used to grow DNA structures with desired architectures. The sticky end links are the weakest parts of such structures. In this work, the strength of sticky end links is studied by computational means. The number of basepairs in the sticky end and the sequence are varied, and the response to tension along the axis of the molecule is evaluated using a full atomistic model. It is observed that, generally, increasing the number of basepairs in the sticky end increases the strength, but the central factor controlling this parameter is the basepair sequence. The sticky ends are divided into two classes of low and high strength. The second class has strength comparable with that of a double stranded molecule with one nick in one of the strands. The strength of the first class is roughly half that of the strong sticky ends. For all strong sticky ends tested, the enhanced stability is associated with the formation of an unusually stable complex composed from two basepairs and two flanking bases of certain sequence. This complex rotates and aligns with the direction of the force allowing significant deformation and providing enhanced strength. This is similar to a mechanism recently suggested to enhance the mechanical stability of an RNA kissing loop from the Moloney murine leukemia virus. The model is tested against experimental structural data for sticky ends and against published simulation results for the stretch of double stranded DNA. The results provide guidance for the design of DNA self-assembled structures and indicate the types of sticky ends desirable if maximizing the strength and stability of these structures is targeted.